Our Milestones

Charting A Path Towards Success.
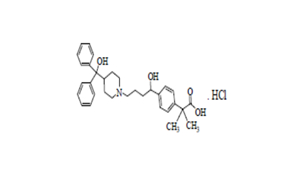
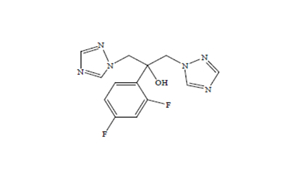
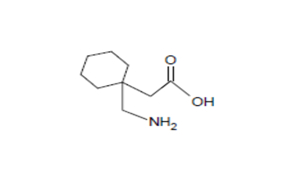
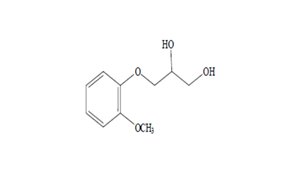
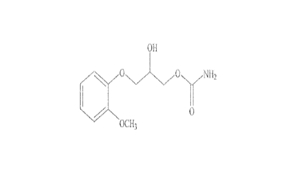
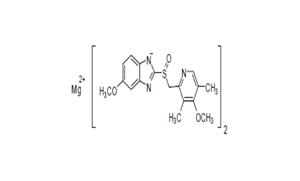
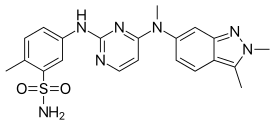
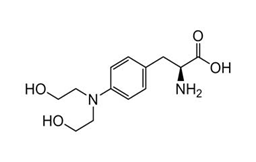
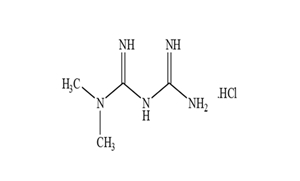
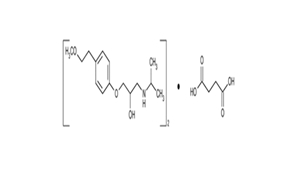
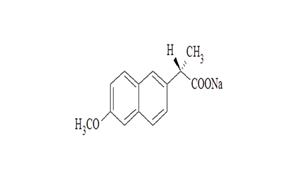
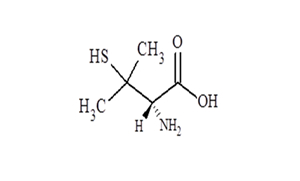
Regulatory Approvals
Revenue (Cr.), FY24
+
Number of Employees
+
Number of Products
Triton Laboratories was formed to produce Paracetamol API at our Bonthapally facility in Hyderabad.

1984
1990
Triton opened its second manufacturing facility at Jeedimetla to produce multiple APIs.
Granules India Private Limited was incorporated.

1991
1993
Granules established its first PFI facility at Jeedimetla.

Granules became a listed company, after having initial public offerings at the Hyderabad Stock Exchange.

1995
2001
Merger of Triton Laboratories with Granules

2003
Granules setsup a new large volume PFI facility in Gagillapur and a wholly owned subsidiary - Granules USA for marketing in the US.

A new Paracetamol plant was built in Bonthapally, Hyderabad.

2005
2008
Granules entered into the Finished Dosage segment.

Granules received US FDA approval for its first Abbreviated New Drug Application (ANDA).
2010
2013
Granules established an API R&D facility in Pragathi Nagar, Hyderabad further to strengthen its vision of being an integrated player.

Granules acquired Auctus Pharma – An API Manufacturing Facility with regulatory approvals

2014
2014
Granules sets up a wholly owned subsidiary in the US; Granules Pharmaceutical to focus on formulation R&D to forward integrate its APIs.

Granules entered the Over-the-Counter business in the US through its own label Granules Consumer Healthcare for OTC products to control the value chain.
2015
2016
Granules laid down the foundation for its Oncology OSD Plant in Visakhapatnam.

To nurture skill development Granules Launched Self Directed Team (SDT) program in Manufacturing Units.

2017
2018
Commenced LEAN SIX-Sigma Program at Granules to inculcate continuous improvement in all areas of business process performance.

Granules entered the front-end business for the sale of Rx Products in the US under the GPI Label.

2019
2020
Granules laid the foundation for the largest single manufacturing site for Multi-Unit Pellet system facility.

Set up GPAK, a 79,000 sq. ft. packaging facility with four packaging suites and a warehouse facility.

2023
2024
Granules Life Sciences (GLS), with a planned FD capacity of 8 bn dosages, successfully commenced operations. Granules CZRO pilot plant commenced operation.

Received approvals for 11 ANDAs.

2020
2019
Filed first oncology DMF & 7 other DMFs | 12 ANDAs & 3 MAs.