FINISHED DOSAGES

Developing technology based challenging formulations
The Finished Dosage R&D facility is completely equipped to accomplish the development of challenging products with complex technologies.We have two R&D sites dedicated for FD development; one in Gagillapur, Hyderabad, India and the other in Chantilly, VA, USA. Over the years, the R&D team has created several distinctive technology-based products with a focus on integration and simplification of processes at scale. R&D at GPI, Virginia focuses on formulation R&D with emphasis on complex formulations across several oral dosage forms. This helps to upgrade our product portfolio with value-added complex generics.
At Granules Finished Dosages R&d, We Possess Capabilities In.
- The development of Rx and OTC products; Immediate Release, Extended Release, Delayed Release, Multi particulate Pellet system based products.
- The development and manufacture of Tablets, Capsules, Press fits, Oral Solutions, Suspensions and Powder for Oral Solutions.
- Method development & validation of Impurities, Reference standard characterization using HPLC. LCMS & GC with validated techniques.
- Scaling up of products between 6Kg to 6000Kg.
- Recognizing Bio Pharmaceutical Classification based requirements and successfully conducting Bioequivalence studies.
KEY HIGHLIGHTS
ANDAs Filed, 55 Approved
EMA- DCP Filed, 02 Approved
Dossiers In Advanced
geographical locations
WE ALSO POSSESS FORMULATION CAPABILITIES IN ONCOLOGY SPACE WITH EXCELLENT KNOW HOW AND ABILITIES TO HANDLE OEB 4 COMPOUNDS UNDER GUIDANCE OF EXPERIENCED TEAM.
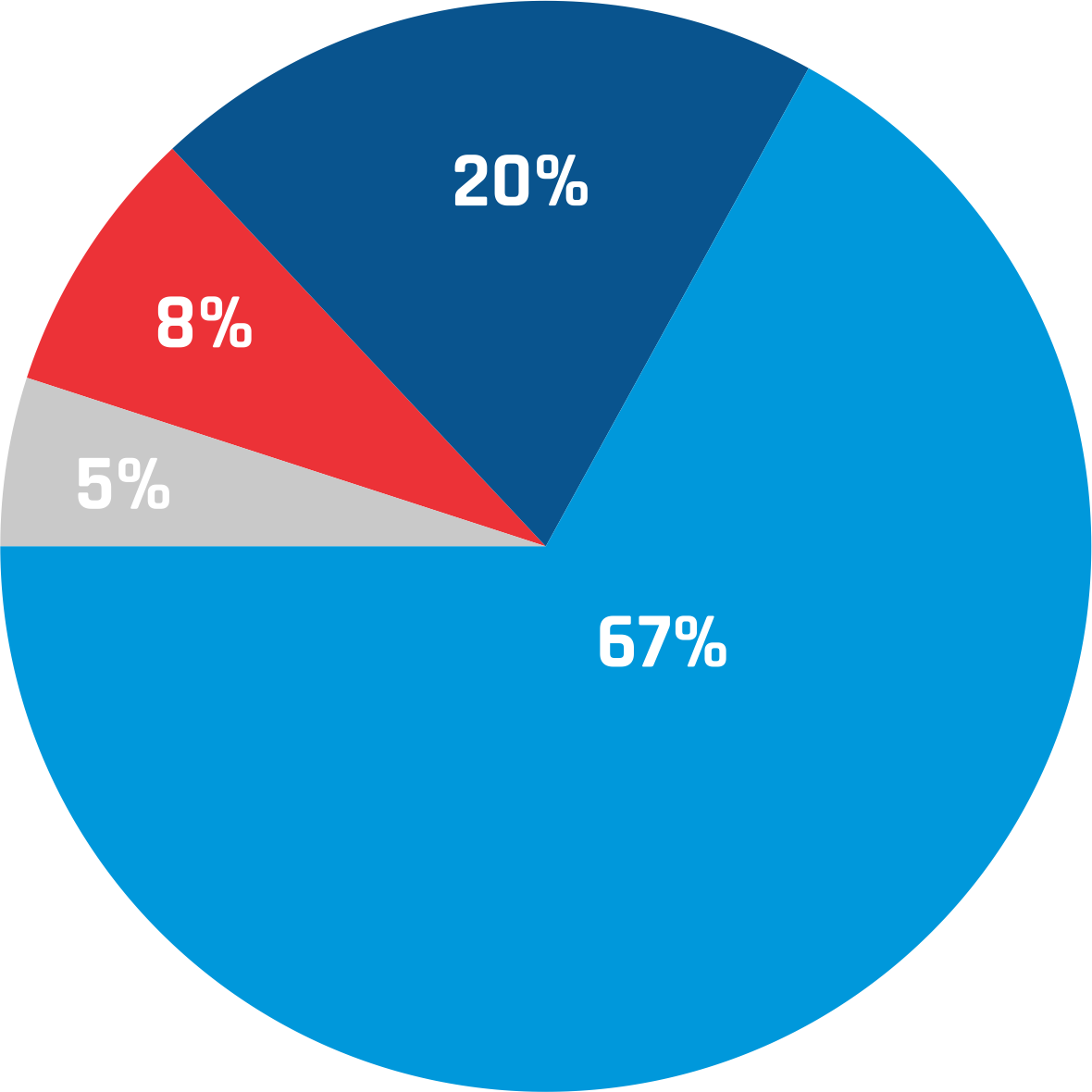
- PHD / ( Post Doc )
Development of complex technology based formulation.
- Other Post Graduation
Support in formulation development & Management | project assistance.
- B. Pharma
Scalability from bench scale to lab Scale at R & D & pilot plant.
- M. Pharma / M.S
Technology based product development pilot & exhibit formulation lead & execution.