| Granules India Limited announces approval of Acetaminophen, Aspirin and Caffeine Tablets (OTC), generic equivalent of Excedrin Migraine Tablets of GlaxoSmithKline Consumer Healthcare |
24-02-2021 |
Download |
| Granules India Limited announces approval of Potassium Chloride ER Capsules USP, generic equivalent of Micro-K ER Capsules of Nesher Pharmaceuticals (USA) LLC |
17-02-2021 |
Download |
| Granules Receives USFDA approval for Potassium Chloride Oral Solution USP |
01-02-2021 |
Download |
| Granules Receives USFDA approval for Metformin Hydrochloride ER Tablets USP, 500 mg and 1000 mg. |
13-01-2021 |
Download |
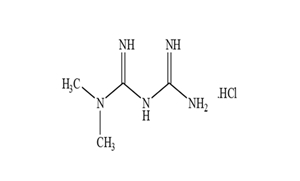
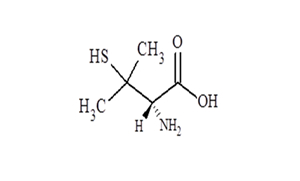
| ANDA approval for Penicillamine Capsules, 250 mg. |
03-12-2020 |
Download |
| Granules India receives US FDA approval for Potassium Chloride Extended Release Tablets USP, 10 mEq (750 mg) and 20 mEq (1500 mg) |
22-10-2020 |
Download |
| Granules receives US FDA approval of Naproxen Sodium and Diphenhydramine Hydrochloride Tablets, 220 mg/25 mg (OTC) |
25-09-2020 |
Download |
| Granules Receives USFDA approval for complex, Attention Deficit Hyperactivity Disorder (ADHD) Drug |
11-09-2020 |
Download |
| Granules Pharmaceuticals, Inc. issues clarification on media reports |
27-07-2020 |
Download |
| Granules Pharmaceuticals, Inc. issues Voluntary Nationwide Recall of Metformin Hydrochloride Extended-Release Tablets USP, 750 mg |
03-07-2020 |
Download |